Rare Dermatology News
Advertisement
Spotlight On
Melanoma
Melanoma is a malignancy of pigment-producing cells (melanocytes) located predominantly in the skin, but also found in the eyes, ears, GI tract, leptomeninges, and oral and genital mucous membranes.
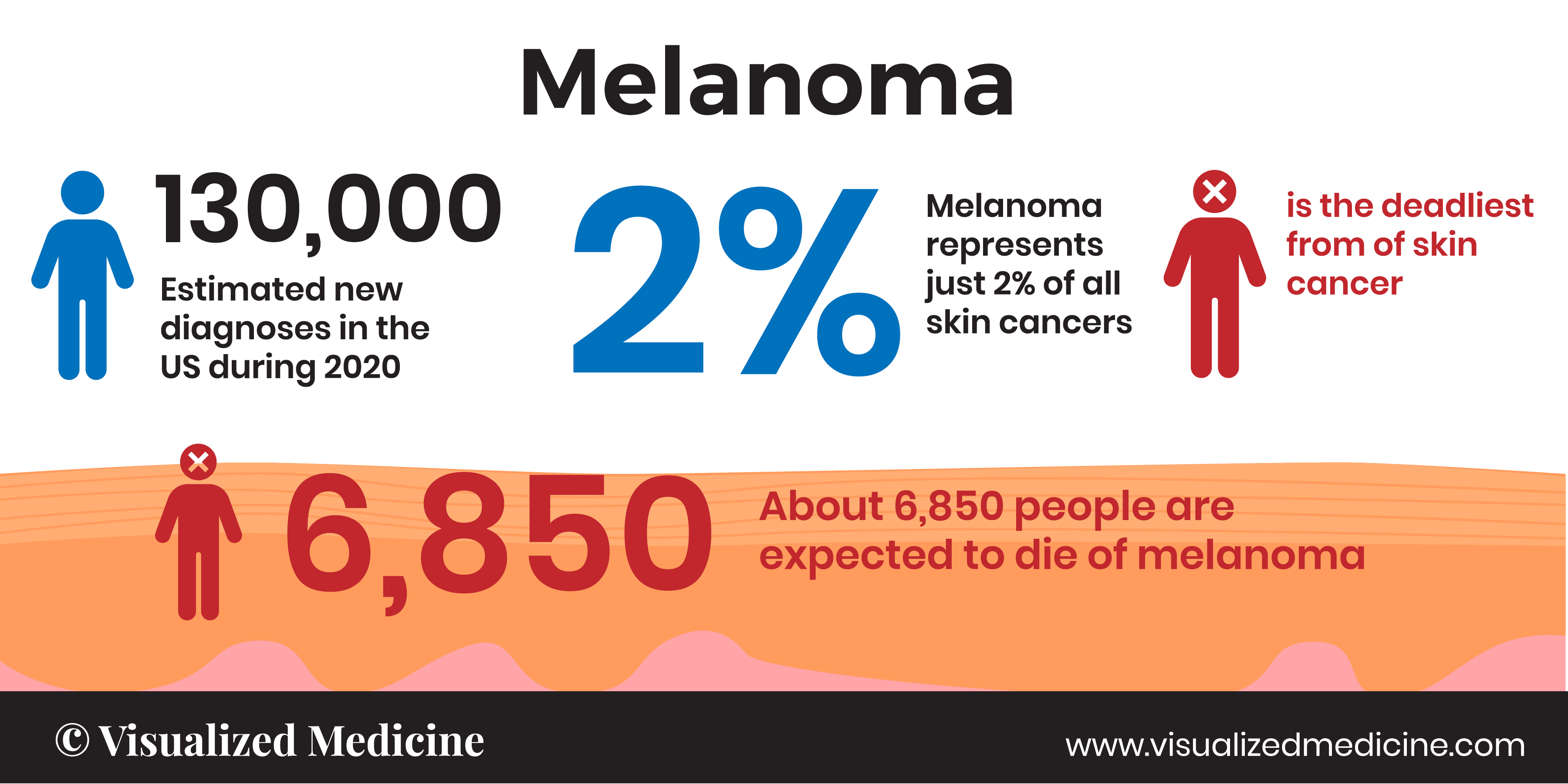
Incidence
Age of Onset

ICD-10
C43.9
Inheritance
This condition does not appear to have a clear pattern of inheritance.
Rare View
Malignant melanoma is the most lethal form of skin cancer. In the last 50 years, its incidence has risen faster than almost any other cancer. Melanoma represents less than 5% of all cutaneous malignancies, but the vast majority of skin cancer deaths. The worldwide incidence of melanoma has risen rapidly over the last 50 years. Its incidence is greatest among fair-skinned populations and in regions of lower latitude.
5 Facts you should know
FACT
New cases of melanoma are increasing annually at almost 6%.
FACT
Melanoma can be colorless.
While it’s true that many melanomas are dark brown to black in color, some melanomas have no color and appear as pink spots or bumps.
FACT
The majority of melanoma-related deaths occur in patients initially diagnosed with early-stage disease.
FACT
The false-negative rate of the SLNB procedure is reported to be between 5-21%.
While SLNB provides prognostic information the procedure has significant limitations including no survival benefit, low sensitivity, and a surgical complication rate of 11%.
FACT
Genomic testing more accurately identifies high-risk melanoma tumors.
When compared with traditional staging, genomic testing can provide a more accurate and personalized assessment of the risk of tumors metastasizing or recurring.
Interest over time
Google searches
Common signs & symptoms
Nevus
Mole
Abnormal hair morphology
Abnormality of the hair
Abnormality of the lymphatic system
Dry skin
Freckling
Abnormality of extrapyramidal motor function
Neoplasm of the breast
Retinopathy
Noninflammatory retina disease
Current treatments
The medication(s) listed below have been approved by the Food and Drug Administration (FDA) as orphan products for treatment of this condition. Learn more orphan products.
Encorafenib + binimetinib
(Brand name: Braftovi + Mektovi) - Manufactured by Array BioPharma, Inc.
FDA-approved indication: June 2018, approved in combination for the treatment of patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, as detected by an FDA-approved test.
Pembrolizumab
(Brand name: Keytruda) - Manufactured by Merck, Sharp & Dohme Corp
FDA-approved indication: Treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor.
Trametinib
(Brand name: Mekinist) - Manufactured by GlaxoSmithKline, LLC
FDA-approved indication: Treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations as detected by an FDA approved test.
Nivolumab
(Brand name: Opdivo) - Manufactured by Bristol-Myers Squibb Co
FDA-approved indication: Treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor. And treatment of patients with melanoma with involvement of lymph nodes or metestatic disease who have undergone complete resection.
Top Clinical Trials
| Title | Description | Phases | Status | Interventions | More Information |
|---|---|---|---|---|---|
| IO102-IO103 in Combination With Pembrolizumab Versus Pembrolizumab Alone in Advanced Melanoma (IOB-013 / KN-D18) | Phase 3, multicenter, international, open-label, randomized, 2-arm trial investigating the safety and efficacy of IO102-IO103 in combination with pembrolizumab as first-line treatment for patients with previously untreated unresectable or metastatic (advanced) melanoma. | Phase 3 | Recruiting | Drug: IO102-IO103|Drug: Pembrolizumab | More Info |
| Efficacy of Daromun Neoadjuvant Intratumoral Treatment in Clinical Stage IIIB/C Melanoma Patients | The trial aims to evaluate the efficacy of Daromun neoadjuvant treatment followed by surgery and adjuvant therapy to improve in a statistically significant manner the recurrence-free survival (RFS) of Stage IIIB/C melanoma patients with respect to the standard of care (surgery and adjuvant therapy). | Phase 3 | Recruiting | Drug: Daromun|Procedure: Surgery|Drug: Adjuvant therapy | More Info |
| A Clinical Trial of Three Study Medicines (Encorafenib, Binimetinib, and Pembrolizumab) in Patients With Advanced or Metastatic Melanoma | The purpose of this study is to learn about the effects of three study medicines (encorafenib, binimetinib, and pembrolizumab) given together for the treatment of melanoma. | Phase 3 | Recruiting | Drug: Encorafenib|Drug: Binimetinib|Drug: Pembrolizumab | More Info |
| Study to Compare Adjuvant Immunotherapy of Bempegaldesleukin Combined With Nivolumab Versus Nivolumab After Complete Resection of Melanoma in Patients at High Risk for Recurrence | The main purpose of this study is to compare the efficacy of bempegaldesleukin plus nivolumab versus nivolumab in patients with completely resected Stage IIIA/B/C/D, or Stage IV cutaneous melanoma who are at high risk for recurrence. | Phase 3 | Recruiting | Biological: Bempegaldesleukin|Biological: Nivolumab | More Info |
| CMP-001 in Combination With Nivolumab Compared to Nivolumab Monotherapy in Subjects With Advanced Melanoma | CMP-001-011 is a Phase 2/3 study of CMP-001 intratumoral (IT) and nivolumab intravenous (IV) compared to nivolumab monotherapy administered to participants with unresectable or metastatic melanoma. | Phase 2|Phase 3 | Recruiting | Drug: CMP-001|Drug: Nivolumab | More Info |
| Neoadjuvant Ipilimumab Plus Nivolumab Versus Standard Adjuvant Nivolumab in Macroscopic Stage III Melanoma | This phase II/III trial studies the side effects of nivolumab and ipilimumab when given together with or without sargramostim and to see how well they work in treating patients with stage III-IV melanoma that cannot be removed by surgery | Phase 3 | Recruiting | Drug: Neoadjuvant ipilimumab + nivolumab|Drug: Adjuvant nivolumab | More Info |
| A Phase II/III Trial of Nivolumab, Ipilimumab, and GM-CSF in Patients With Advanced Melanoma | This phase II/III trial studies the side effects of nivolumab and ipilimumab when given together with or without sargramostim and to see how well they work in treating patients with stage III-IV melanoma that cannot be removed by surgery. | Phase 2|Phase 3 | Recruiting | Biological: Ipilimumab|Biological: Nivolumab|Biological: Sargramostim | More Info |
| Evaluation of the Length of Treatment With PD-1/PD-L1 Inhibitors in Patients With Advanced Solid Tumors | The investigators are proposing a trial that will randomize patients who have disease stability to stop treatment at 1 year or continue treatment until disease progression. The investigators anticipate that the results of this study will answer questions regarding the optimal duration of treatment. therapy. | Phase 3 | Recruiting | Drug: Continue PD-1/PD-L1 Inhibitors treatment|Other: Discontinue PD-1/PD-L1-1 inhibitor | More Info |
Top Treatments in Research
| Agent | Class/Mechanism of Action | Development Status | Company | Clinical Studies | More Information |
|---|---|---|---|---|---|
| IO102-IO103/Pembrolizumab | IO102-IO103 is an investigational cancer vaccine designed to target the immunosuppressive mechanisms mediated by key immunosuppressive proteins such as Indoleamine 2,3-dehydrogenase (IDO) and PD-L1.Pembrolizumab (Keytruda) is a humanized monoclonal antibody to programmed cell death receptor 1 (PD-1), which results in an increased immune reactivity that can break tolerance and is used in the immunotherapy of cancer. | Phase 3 | IO Biotech | More Info | More Info |
| Daromun | Daromun (Philogen) is a combination of Darleukin, a human vascular targeting monoclonal antibody (L19) fused to interleukin-2 (IL-2), and Fibromun, the L19 antibody linked to tumor necrosis factor (TNF). | Phase 3 | Philogen | More Info | More Info |
| Encorafenib/Binimetinib/Pembrolizumab | Encorafenib is a small molecule BRAF inhibitor that targets key enzymes in the MAPK signaling pathway. Binimetinib is an uncompetitive, small molecule inhibitor of selective mitogen-activated protein kinase (MEK1/2). .Pembrolizumab (Keytruda) is a humanized monoclonal antibody to programmed cell death receptor 1 (PD-1), which results in an increased immune reactivity that can break tolerance and is used in the immunotherapy of cancer. | Phase 3 | Pfizer | More Info | More Info |
| Bempegaldesleukin/Nivolumab | Bempegaldesleukin is a PEGylated interleukin-2 (IL-2) acting as a CD122-preferential IL-2 pathway agonist designed to activate and proliferate CD8 + T cells and NK cells. Nivolumab is a human recombinant monoclonal immunoglobulin G4 antibody to the programmed cell death receptor-1 (PD-1) which has distinctive immunomodulatory activity and is used in cancer immunotherapy. | Phase 3 | Nektar Therapeutics | More Info | More Info |
| CMP-001/Nivolumab | Anti-Qβ-coated CMP-001 induces IFN-α production by pDCs which has secondary effects on a variety of cells including monocytes. Nivolumab is a human recombinant monoclonal immunoglobulin G4 antibody to the programmed cell death receptor-1 (PD-1) which has distinctive immunomodulatory activity and is used in cancer immunotherapy. | Phase 2|Phase 3 | Checkmate Pharmaceuticals | More Info | More Info |
| Neoadjuvant ipilimumab + nivolumab/Adjuvant nivolumab | Ipilimumab, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4. Nivolumab is a human recombinant monoclonal immunoglobulin G4 antibody to the programmed cell death receptor-1 (PD-1) which has distinctive immunomodulatory activity and is used in cancer immunotherapy. | Phase 3 | The Netherlands Cancer Institute | More Info | More Info |
| Ipilimumab/Nivolumab/Sargramostim | Ipilimumab, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4. Nivolumab is a human recombinant monoclonal immunoglobulin G4 antibody to the programmed cell death receptor-1 (PD-1) which has distinctive immunomodulatory activity and is used in cancer immunotherapy. | Phase 2|Phase 3 | National Cancer Institute (NCI) | More Info | More Info |
| Continue PD-1/PD-L1 Inhibitors treatment/Discontinue PD-1/PD-L1-1 inhibitor | PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. | Phase 3 | Antoinette J Wozniak, University of Pittsburgh | More Info | More Info |